
When clinical trials become a normal part of care options for patients with unmet needs, both researchers and patients will reap exponential rewards.
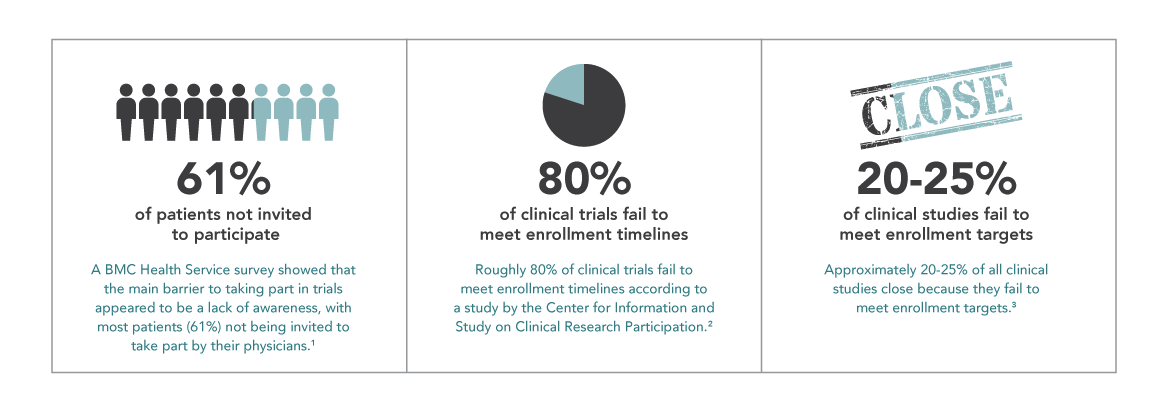
“Where are the patients?” Clinical trials hinge on recruitment, and enrollment issues are a perpetual challenge. Research estimates that, on average, around 20-25% of trials across all phases and therapeutic areas are terminated due to patient enrollment problems, and roughly 80% of clinical trials fail to meet enrollment timelines. Since research also shows that more than 50% of all eligible patients invited to participate in trials say yes, we can deduce that not enough patients are being given an informed opportunity for participation. Recent studies show that in oncology, for example, only 8% of cancer patients in the U.S. participate in clinical trials. It’s a case of “many are willing, but few are invited.”
NIH reports that 77% of patients who participate in a trial learned about it from their healthcare provider. These are the gatekeepers that patients trust. But a Harris Interactive Study showed that 85% of patients were either unaware or unsure that participation in a clinical trial was an option at the time of diagnosis, while 75% of these same patients said they would have been willing to enroll had they known it was possible. A Research America poll found that 80% of 1,000 respondents claim a physician’s recommendation is an important factor in deciding to participate, yet only 22% of patients said a healthcare professional has talked to them about it. Clinical trial advocacy is clearly not a part of the standard of care for a great percentage of healthcare providers. In order to increase the potential patient pool for trials and to offer more patients the option to participate, we must move towards integrating research and clinical care in all provider settings.
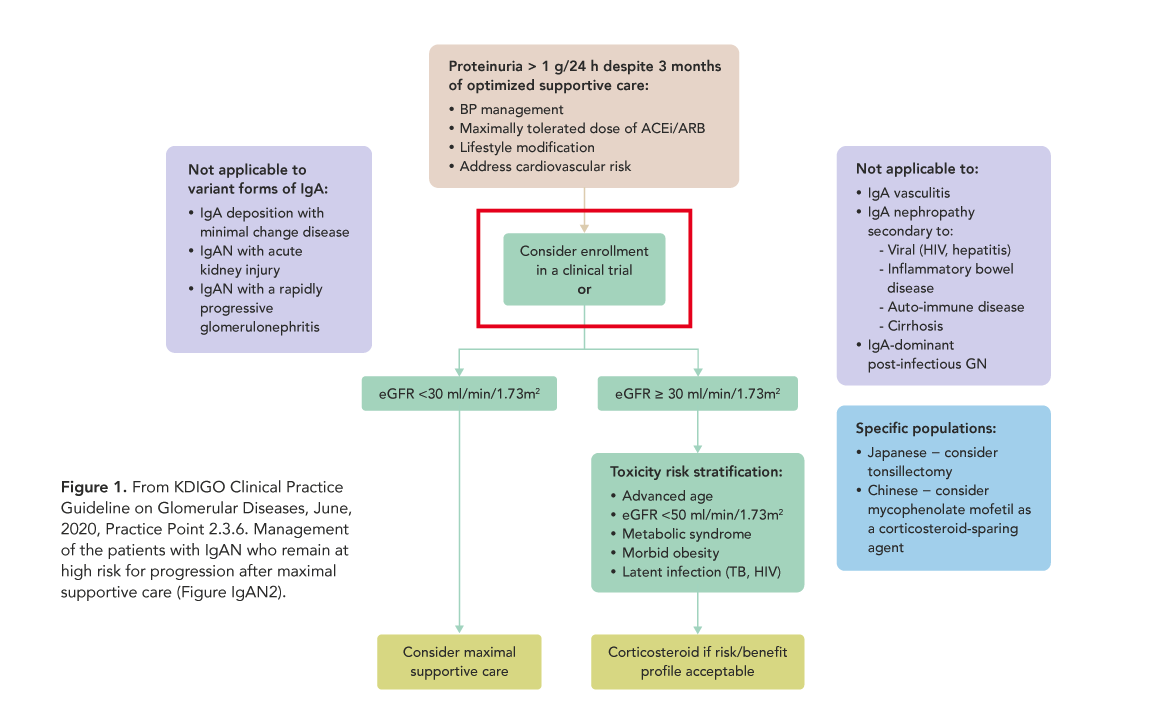
This concept is beginning to be more widely promoted and logistically more realistic. For the first time ever, recent KDIGO Clinical Practice Guidelines on Glomerular Diseases include that in particular cases, the treatment option most recommended is to enroll the patient into an existing clinical trial. (Fig. 1) And some of the barriers to recruiting “outside” of research centers are becoming less problematic, as the pandemic situation has accelerated acceptance of remote participation for many elements of trials. As healthcare evolves, clinical trial advocacy as a part of routine clinical care for all patients everywhere should be one of our goals.
Physicians in all therapeutic areas and in all types of practices should become more proactive in assessing at what point in a patient’s healthcare journey that enrolling in a clinical trial would be a good option in their treatment. Trials represent another resource for physicians to give patients more favorable outcomes and to improve their overall healthcare experience, as patients enrolled in clinical trials generally report that their level of care is better when enrolled. Embedding clinical trials into patient care will change trials from the current “two-tiered” structure where clinical trials are a separate “system” into a more seamless and integrated process that makes research more robust, more relevant to day-to-day patient care and more impactful in the real world of clinical practice. Both researchers and patients will see exponential benefits in a shorter period of time and with greater efficiency.
Bridging the gap between research and clinical care begins with including hypothesis testing in the education of our young doctors — teaching them to think like trialists and extending their skills beyond prescribers. When clinical trials become a normal part of care options for patients with unmet needs, available patient populations will not only be larger, but better targeted to specific research goals, optimizing both recruitment and patient retention. More patients in more settings will be able to benefit from research efforts and receive enhanced delivery of care. Trials will be more cost-effective, able to meet tighter timelines and, most importantly, have a greater impact on standards of clinical care for all patients everywhere.
It will take time and effort to affect these changes for the majority of physicians and patients globally but the rewards will be worth the work, ultimately leading to getting the best treatment options to as many people as possible in the shortest amount of time. Trials will be more efficient and cost effective. Research will include a more diverse and representative population. Global healthcare will be advanced. And researchers won’t have to ask “Where are the patients?” when getting a new lifesaving clinical trial underway.



