Kidney & Metabolic
George Clinical has established a reputation as the clinical research organization of choice for conduct of renal trials.
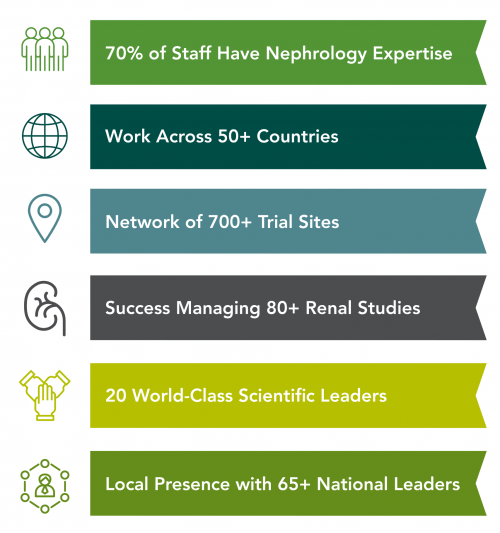
Facilitated by a kidney site network considered to be one of the strongest in the world, especially in the Asia Pacific region, George Clinical owns an enviable position as a trusted partner in kidney and metabolic clinical research around the globe. Our scientific expertise collaborates with sponsors to develop new strategies for the prevention and management of renal and metabolic disease specifically including dialysis, chronic kidney disease (CKD), focal segmental glomerulosclerosis (FSGS), diabetic kidney disease, acute kidney injury and IgA nephropathy as well as the utility of genetics in drug development and discovery.
George Clinical leverages the in-depth expertise of our scientific leadership to design effective studies, select the best sites from more than 20 countries internationally, and accelerate recruitment. Our scientific leaders generate strong evidence to implement real change in treatments, clinical practice and policy.
Working with George Clinical means studies activate quicker, recruit thoroughly, and meet or exceed budgetary expectations through the continuous support of dedicated, regionally savvy, cross-functional teams.
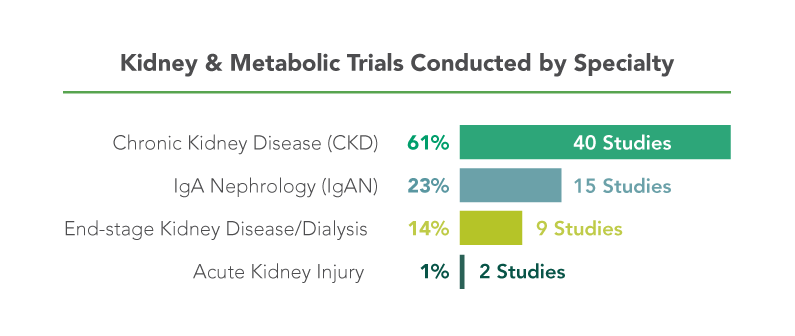
George Clinical has earned a reputation as the leading kidney and metabolic CRO through a broad resume of landmark studies.

Dr. Hettiarachchi specializes in clinical trial operational strategy for recruitment, retention and successful trial delivery. An experienced clinical trial specialist, he has nearly three decades of clinical research experience in academic research, CROs, Pharma and biotech. He has managed many global clinical trials from start-up to CSR in various therapeutic areas, specializing in pivotal renal trials (IgAN, CKD, DKD, etc.) and in supporting operational teams in APAC and globally. He is exploring opportunities for clinical trials in new countries where there is high patient availability.
Accelerating kidney research and improving the quality of care for patients with kidney disease requires innovative thinking and challenging the status quo. Our team of world-class scientific leaders and operational experts connect key opinion leaders, investigators, research sites, patients, regulators and clients with a common purpose and an innovative operational platform for conducting kidney clinical trials. The GKPTN is endorsed by the International Society of Nephrology and aims to work with kidney disease networks globally. Collaboratively, we will take kidney research to the next level across the globe. By bringing patients, established sites and trials closer together, GKPTN aims to strengthen the global kidney disease research community’s capacity to facilitate more rapid translation of new interventions from research into practice.