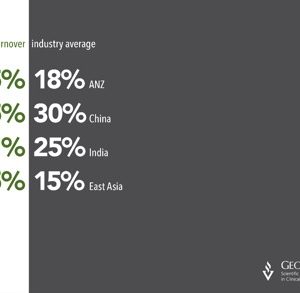
Link between birthweight and cancer confirmed by new research
New research from the International Childhood Cancer Cohort Consortium (I4C) confirms that childhood cancer is linked to birthweight. Childhood cancer appears to be slowly rising, at a rate of approximately 1% per year in developed countries. The I4C is an alliance of several large-scale studies of children that pools data on factors in relation to […]
Read More